Informatics Consult
For any of these projects, you’ll need data. So where do you get your data and how do you do that while ensuring you are following the rules and regulations? As you are aware, there are federal and state laws around accessing data, use of data, and storage of data. Because this can be very confusing, we’ve pull together the following flows to help you
- Clinical Trial
DATA SOURCES
- Patient
- Epic Medical record
- Survey/Behavioral
- Biospecimen (current/prospective collection)
- QOL
- Observational requiring access to patient/records
- Qualitative
- Registry that addresses a specific question/problem
- Any non-interventional study that requires direct access to a patient or access to patients MRN to collect data for future clinical services/outcomes
DATASOURCES
- Patient
- Epic Medical Record
- Biospecimen
- Chart review
- Observational not requiring access to patients/records
- Student projects
- NHSR
- Any non-interventional study that does not require direct access to a patient or access to patients MRN to collection data on future clinical services/outcomes
DATA SOURCES
- TriNetX
- Epic Comos
If you are consenting study participants, then your access to data is via patient encounter or Epic. You will receive approval for this data pathway by IRB approval.
If you are not consenting participants, there are specific pathways to obtain data: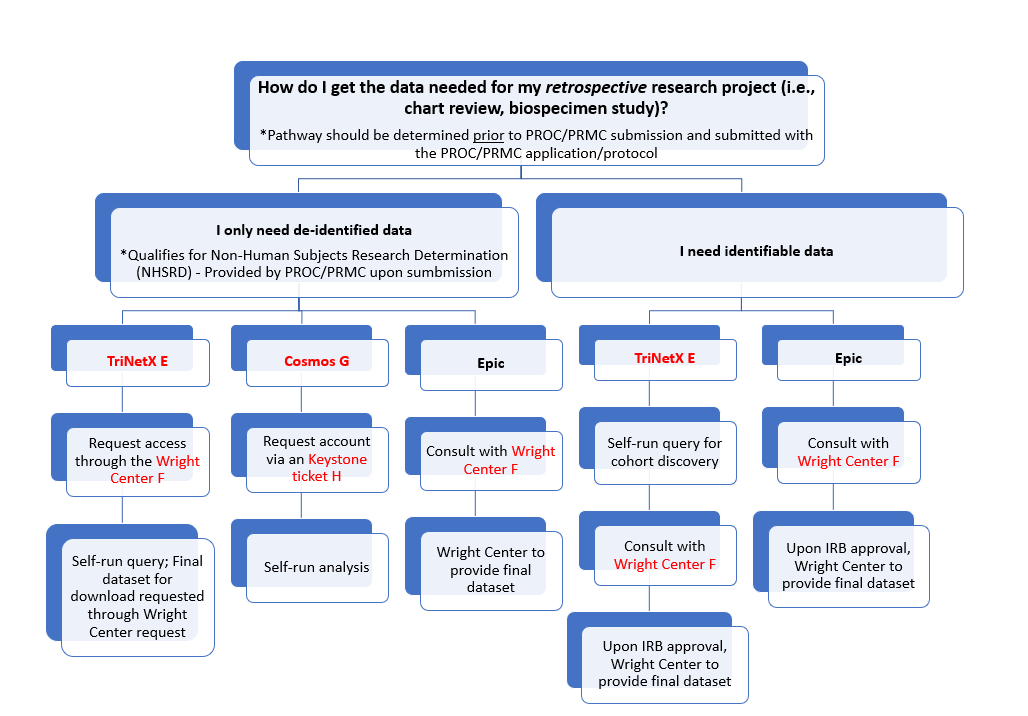
An Honest Broker is a neutral third party who collects and provides de-identified patient data or specimens to researchers, acting as an intermediary to ensure confidentiality and privacy by removing any identifiable information while facilitating access to necessary research materials.
-
- Honest Brokers act on behalf of VCUHS to provision datasets for research purposes;
- Are not part of the study team; and
- Limit the amount of identifiable information a study team needs to access during data collection
Use of an Honest Broker is required by VCU Health’s COMP-014 policy to adhere to HIPAA requirements. The primary team of Honest Brokers set within VCU’s Wright Center (see https://cctr.vcu.edu/support/informatics/ for more information.)
If you need identifiable data, there are two pathways. The first is via TriNetX. You can obtain access to TriNetX by submitting a request to the Wright Center. When you have access to the system, you will run a self-directed query for cohort discovery. Upon completion, you should schedule a consult with the Wright Center Honest Brokers who will, upon IRB approval, provide you with a final database. The second pathway is via Epic. This route requires a consult with the Honest Brokers in the Wright Center who will provide you with a final dataset.
If you have any questions about data acquisition, you can always reach out to Compliance email, Wright Center email, or clinical.research@vcuhealth.org
Now that you know if your project is Research or QI and you know the type of data you need, the next step is to submit to the applicable PROC